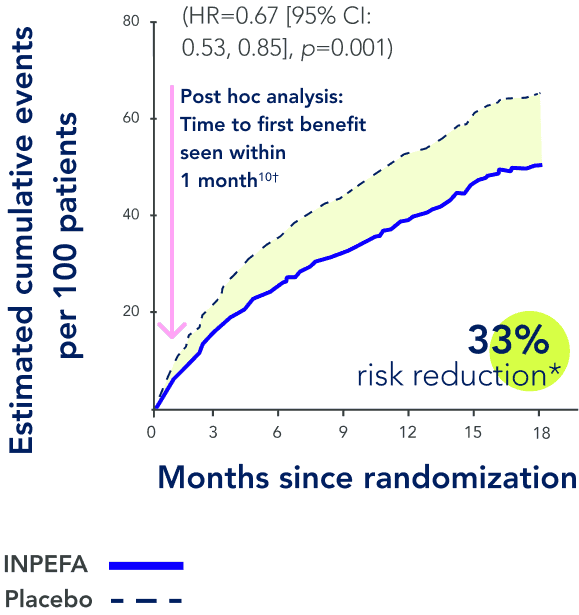
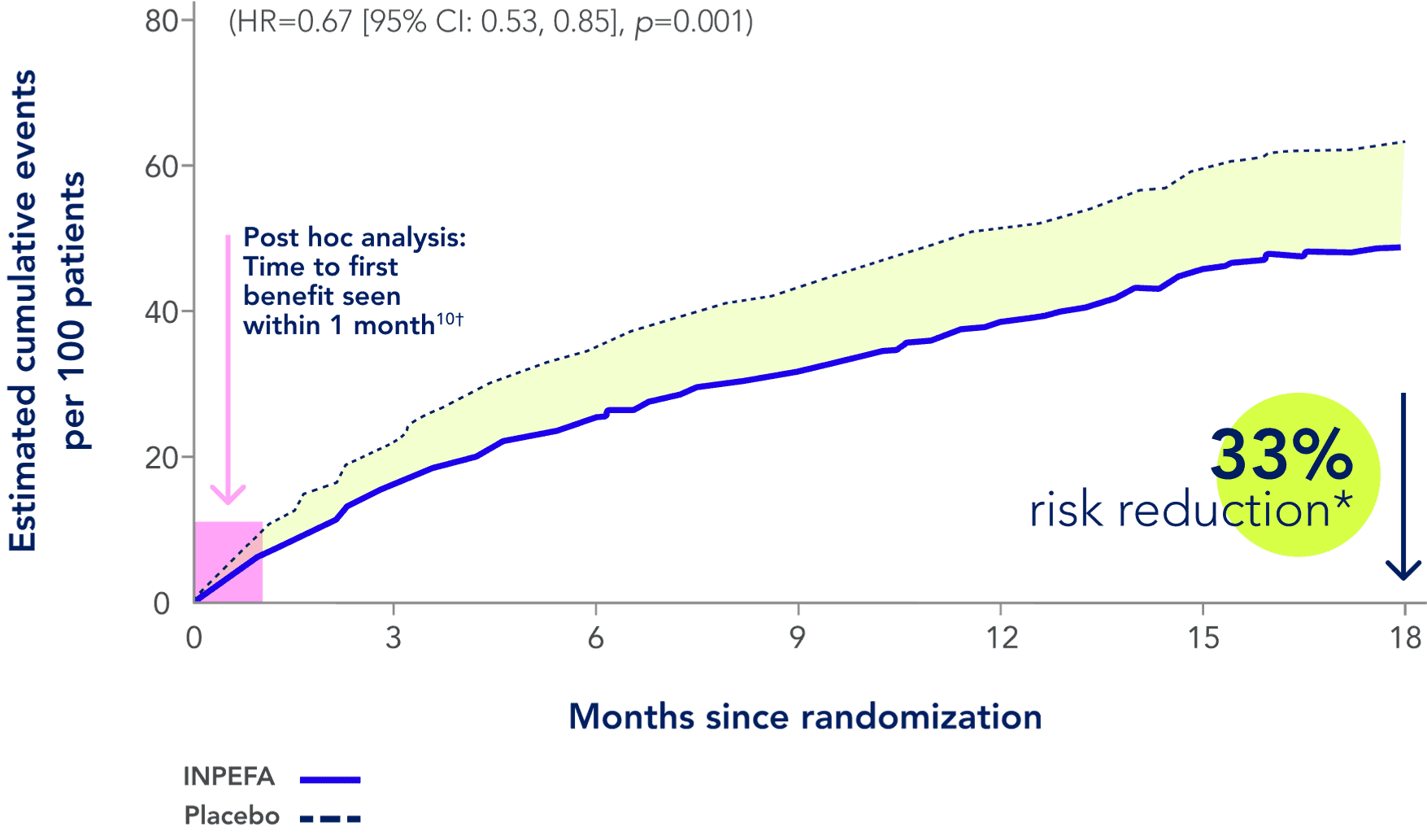
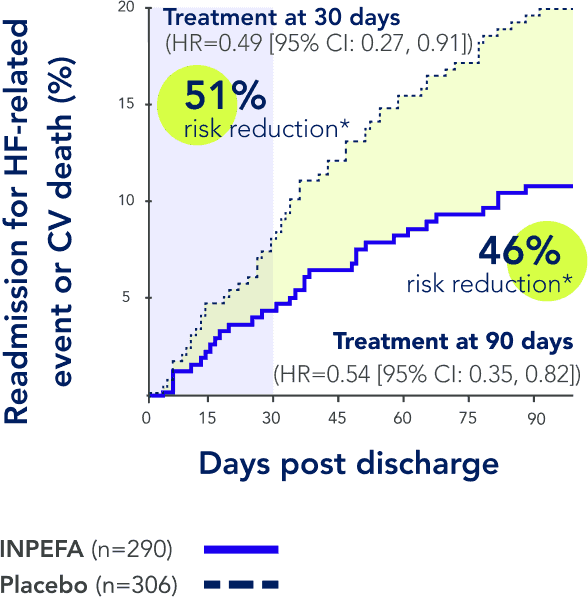
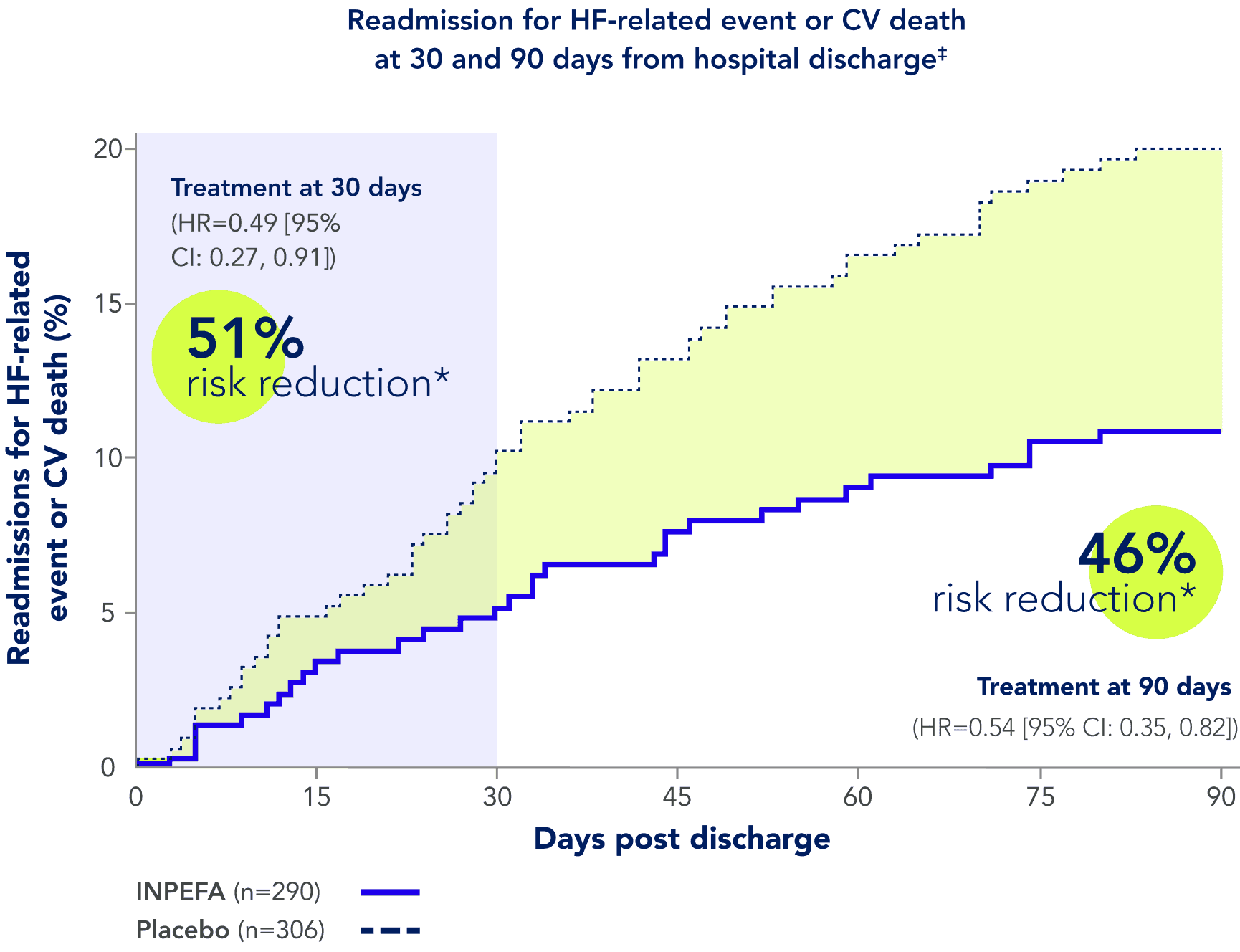
References:
1. INPEFA [prescribing information]. Lexicon Pharmaceuticals, Inc.; 2024. 2. Maddox, T, Januzzi, J, Allen, L. et al. 2024 ACC Expert consensus decision pathway for treatment of heart failure with reduced ejection fraction: a report of the American College of Cardiology solution set oversight committee. J Am Coll Cardiol. 2024;83(15):1444‑1488. doi.org/10.1016/j.jacc.2023.12.024 3. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American Colege of Cardiology/American Heart Association joint committee on clinical practice guidelines [published correction appears in Circulation. 2022 May 3;145(18):e1033]. Circulation. 2022;145(18):e895-e1032. doi:10.1161/CIR.0000000000001063 4. Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384(2):117-128. doi:10.1056/ NEJMoa2030183 5. Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387(12):1089‑1098. doi:10.1056/NEJMoa2206286 6. Voors AA, Angermann CE, Teerlink JR, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022;28(3):568‑574. doi:10.1038/s41591‑021‑01659‑1 7. FARXIGA (dapagliflozin) [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2023. 8. Verma S, Anker SD, Butler J, Bhatt DL. Early initiation of SGLT2 inhibitors is important, irrespective of ejection fraction: SOLOIST-WHF in perspective. ESC Heart Fail. 2020;7(6):3261-3267. doi:10.1002/ehf2.13148 9. Pitt B, Bhatt DL, Szarek M, et al. Effect of sotagliflozin on early mortality and heart failure-related events: a post hoc analysis of SOLOIST-WHF [published correction appears in JACC Heart Fail. 2023 Sep;11(9):1288]. JACC Heart Fail. 2023;11(8 Pt 1):879-889. doi:10.1016/j.jchf.2023.05.026 10. Verma S, Bhatt DL, Dhingra NK, et al. Time to benefit with sotagliflozin in patients with worsening heart failure. J Am Coll Cardiol. 2023;81(15):1546-1549. doi:10.1016/j.jacc.2023.02.022